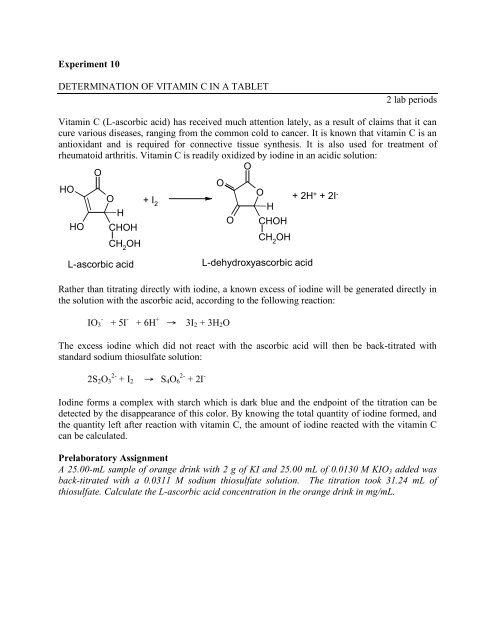
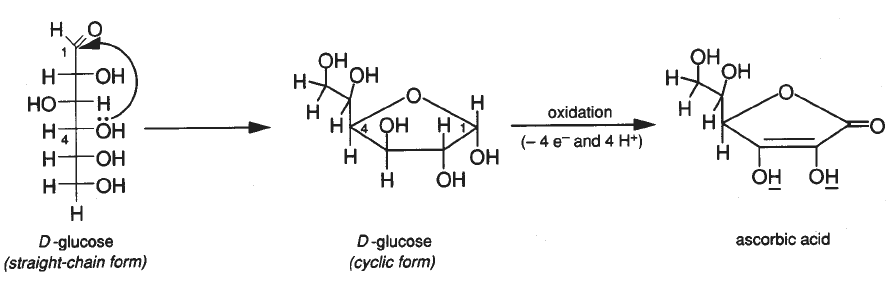
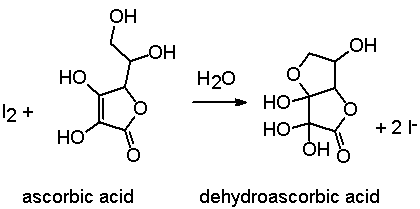
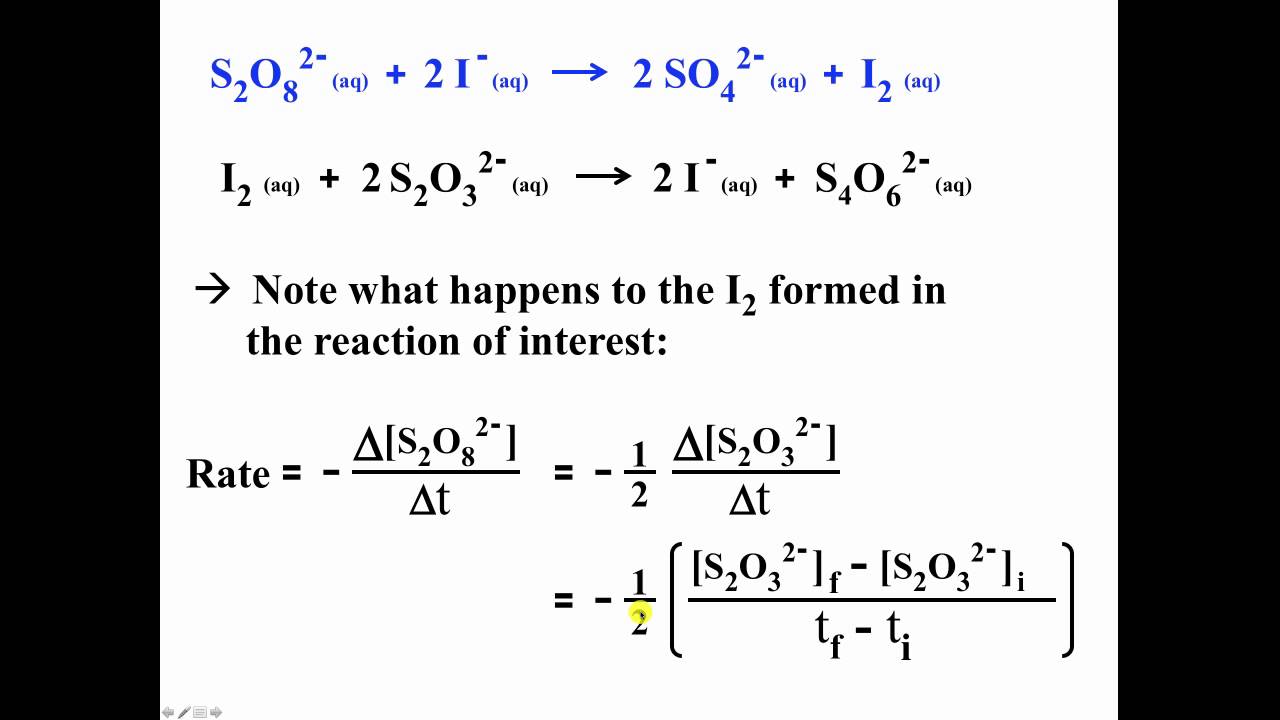
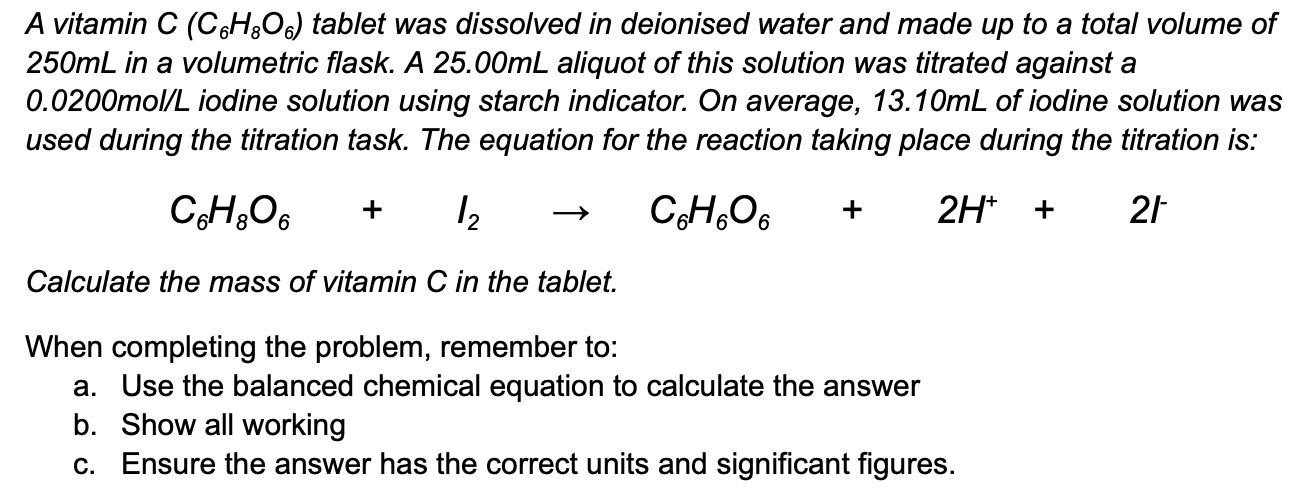
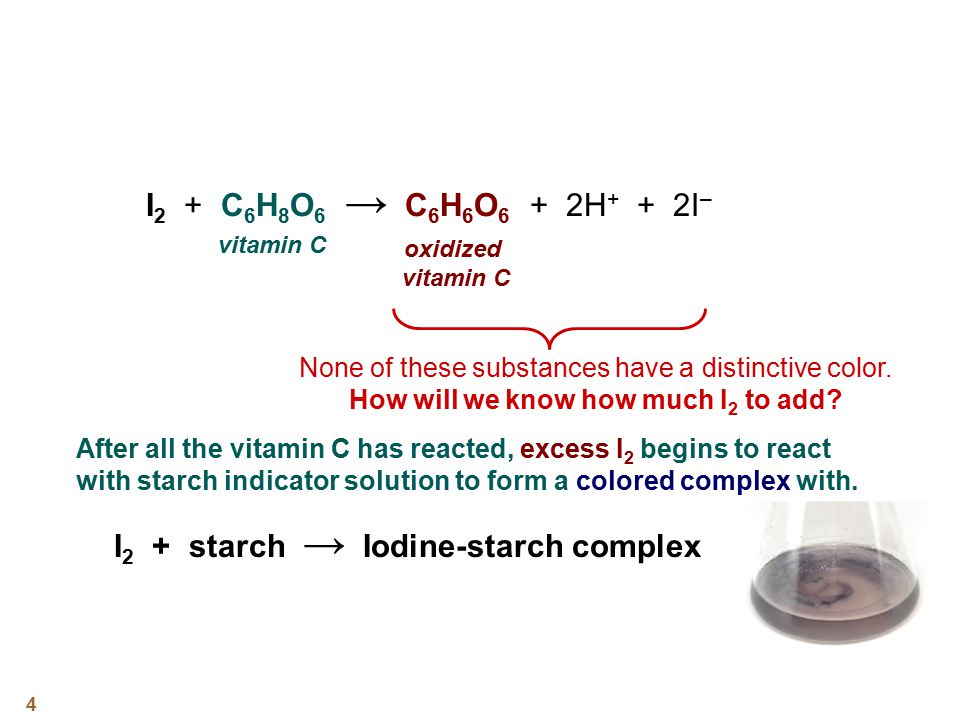
Applied Sciences | Free Full-Text | Determination of Vitamin C in Foods Using the Iodine-Turbidimetric Method Combined with an Infrared Camera

Using a classical method of vitamin C quantification as a tool for discussion of its role in the body - ScienceDirect
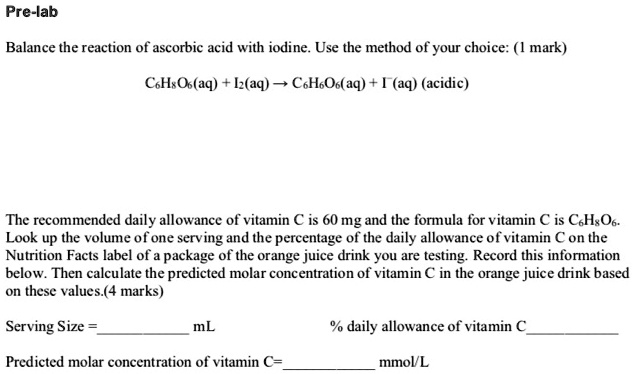
SOLVED: Pre-lab Balance the reaction of ascorbic acid with iodine. Use the method of your choice: mark) CoH3O3(aq) + I2(aq) â†' CoH2O3(aq) + I2(aq) (acidic) The recommended daily allowance of vitamin C

redox - What is the role of various additives in a titration of vitamin C with N-bromosuccinimide - Chemistry Stack Exchange
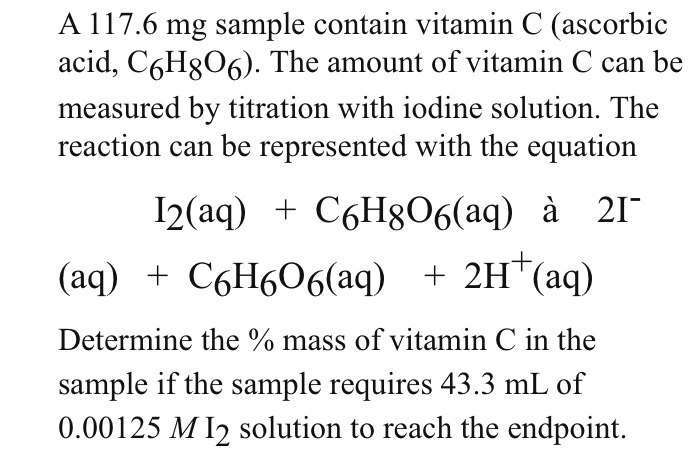
![SOLVED: 6-B Vitamin C (ascorbic acid) from foods can be measured by titration with [5 - C6H8O6 + I2 + 2H2O â†' C6H6O6 + 2HI]. Ascorbic acid reacts with triiodide to form SOLVED: 6-B Vitamin C (ascorbic acid) from foods can be measured by titration with [5 - C6H8O6 + I2 + 2H2O â†' C6H6O6 + 2HI]. Ascorbic acid reacts with triiodide to form](https://cdn.numerade.com/ask_images/99732dc17784450abeefac01d46a12cd.jpg)
SOLVED: 6-B Vitamin C (ascorbic acid) from foods can be measured by titration with [5 - C6H8O6 + I2 + 2H2O â†' C6H6O6 + 2HI]. Ascorbic acid reacts with triiodide to form

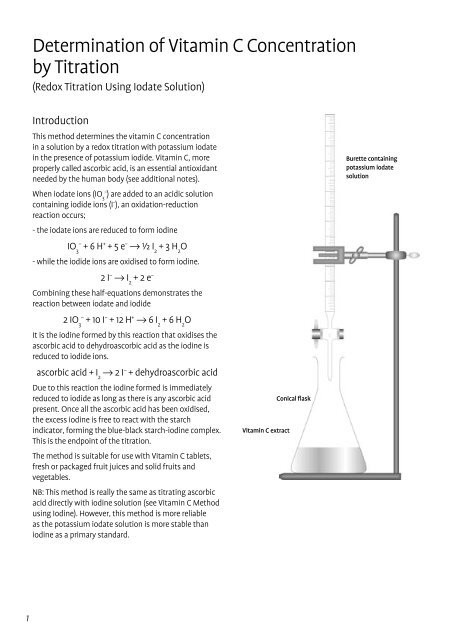
Experiments for redox reactions of ascorbic acid and iodine solutions.... | Download Scientific Diagram
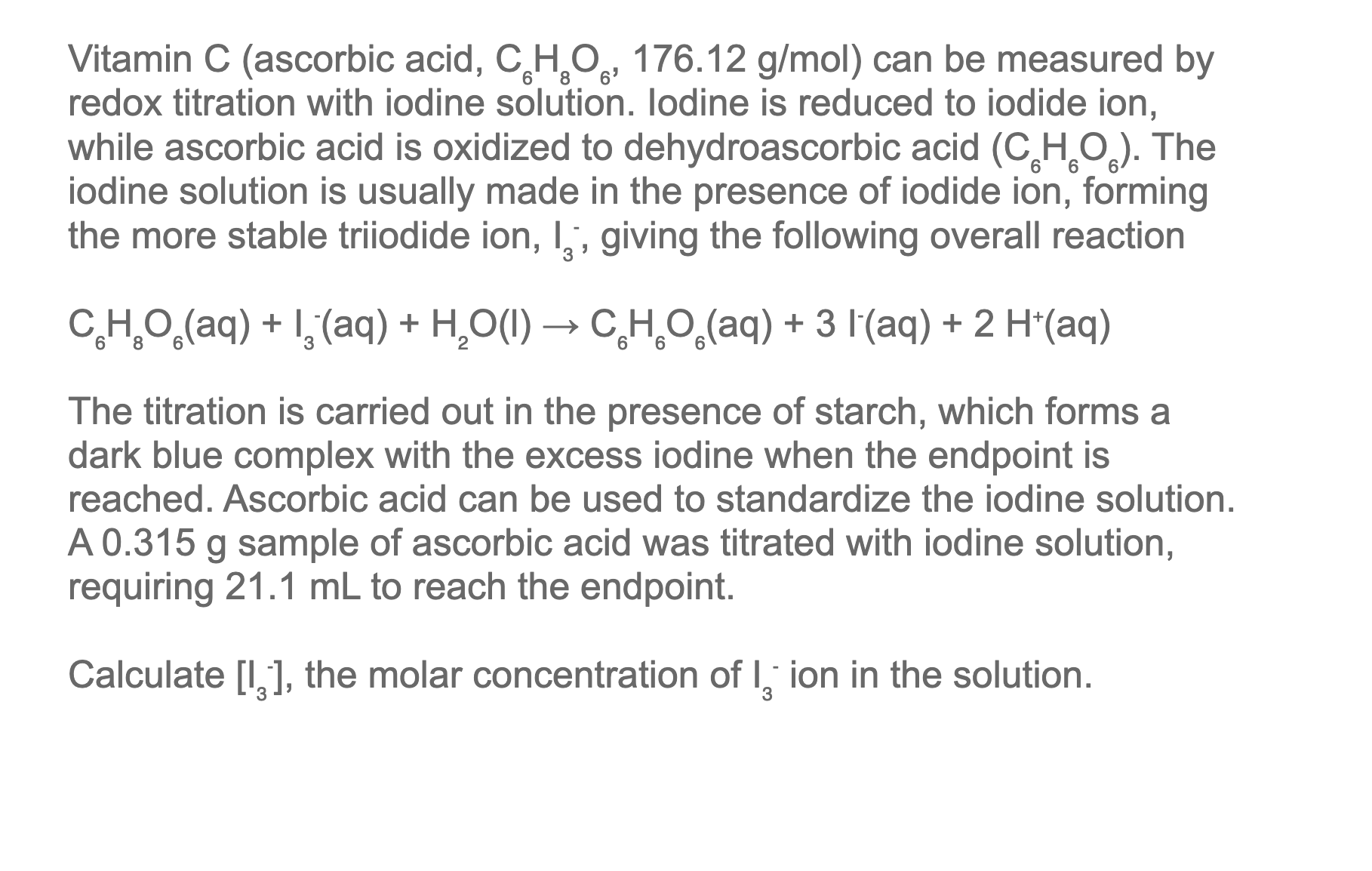
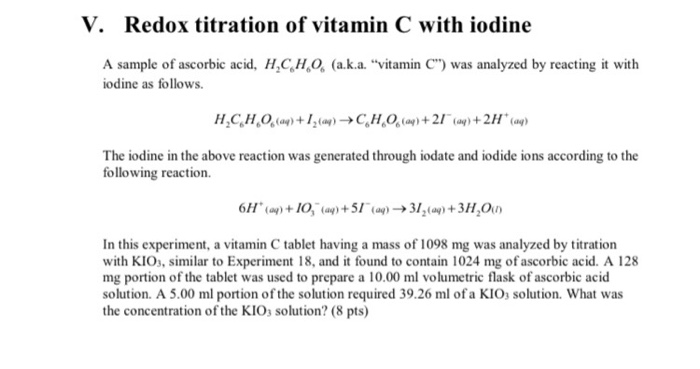
SOLVED: Vitamin C (ascorbic acid, C6H8O6, 176.12 g / mol ) can be measured by redox titration with iodine solution. lodine is reduced to iodide ion, while ascorbic acid is oxidized to