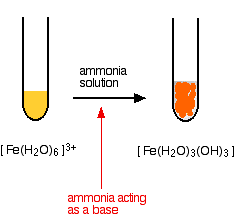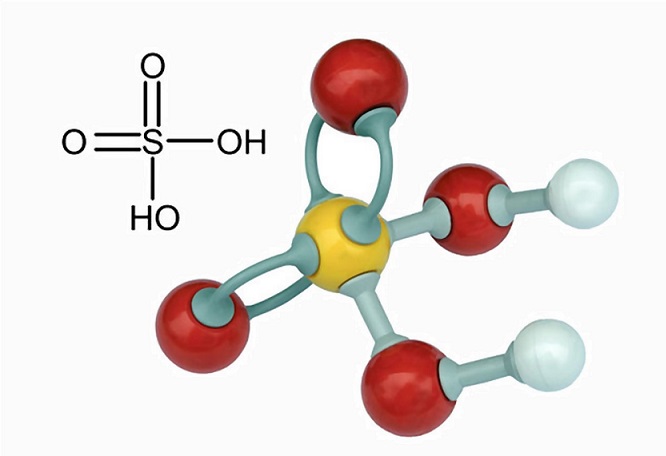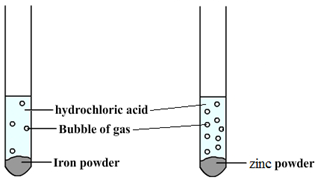
2:15 understand how metals can be arranged in a reactivity series based on their reactions with: water and dilute hydrochloric or sulfuric acid - TutorMyself Chemistry

Effect of sulfuric acid concentration on iron dissolution and final pH.... | Download Scientific Diagram

The process of pyrite weathering in a deep metal mine. Four general... | Download Scientific Diagram

Sulfuric Acid LO: Outline uses and reactions involving Sulfuric Acid Starter: What is an acid? - ppt download
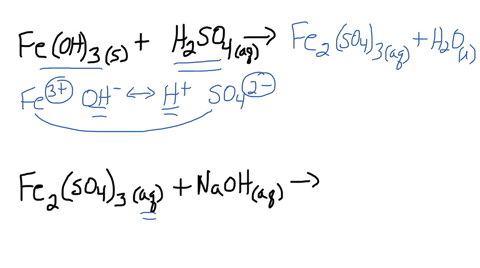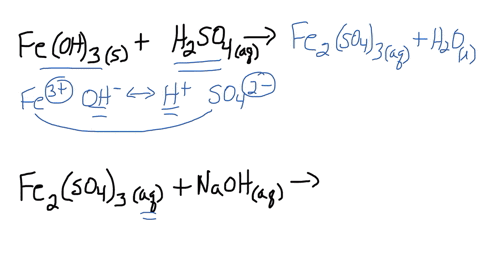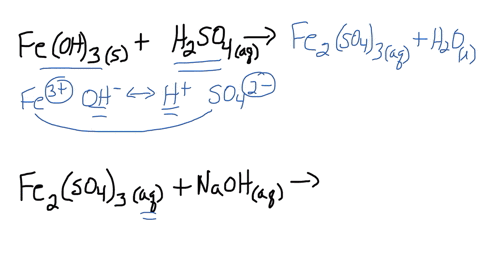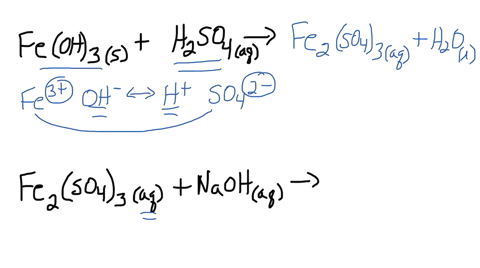
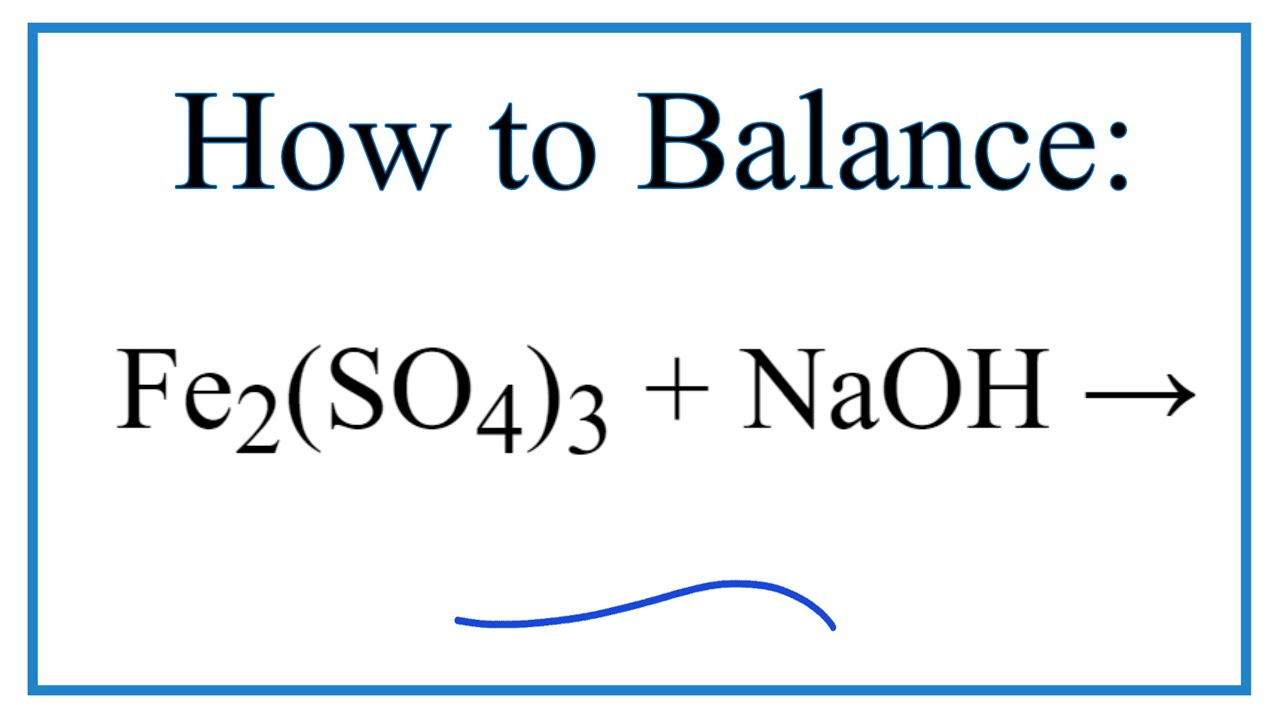
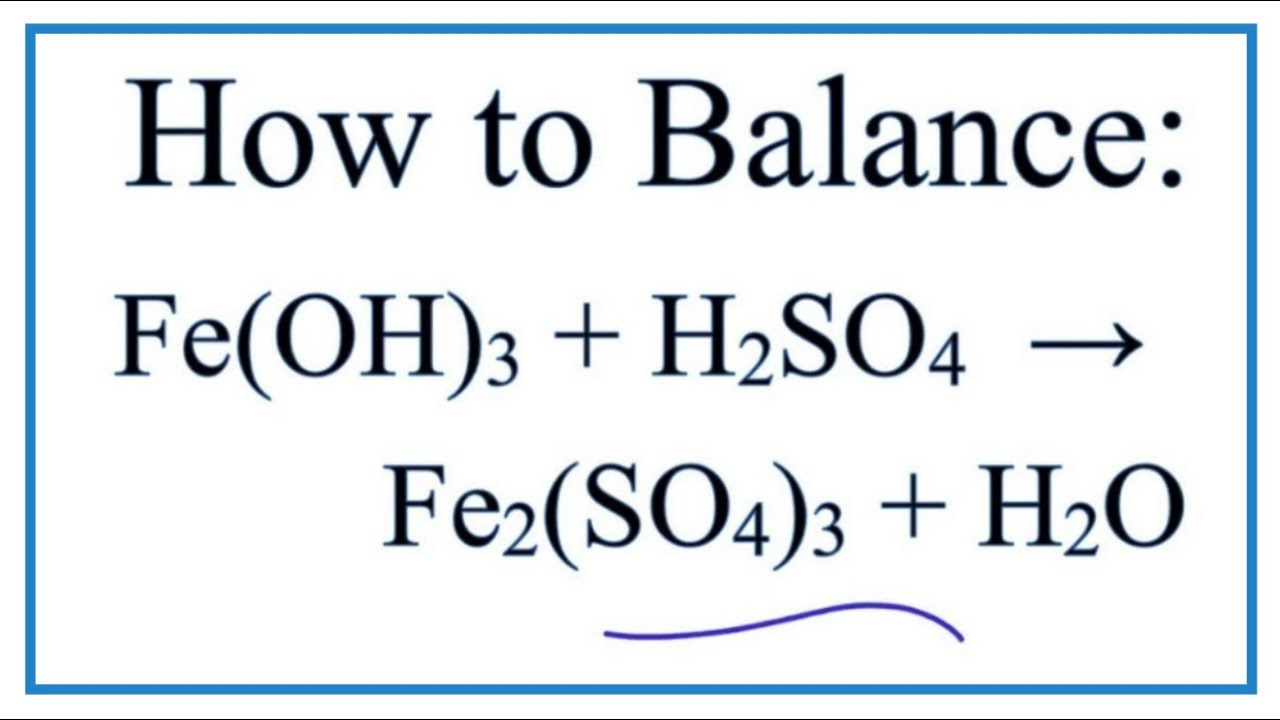
SOLVED: Iron(III) sulfate is made in industry by the neutralization reaction between solid iron(III) hydroxide and aqueous sulfuric acid. The iron(III) sulfate is then added with sodium hydroxide to municipal water in
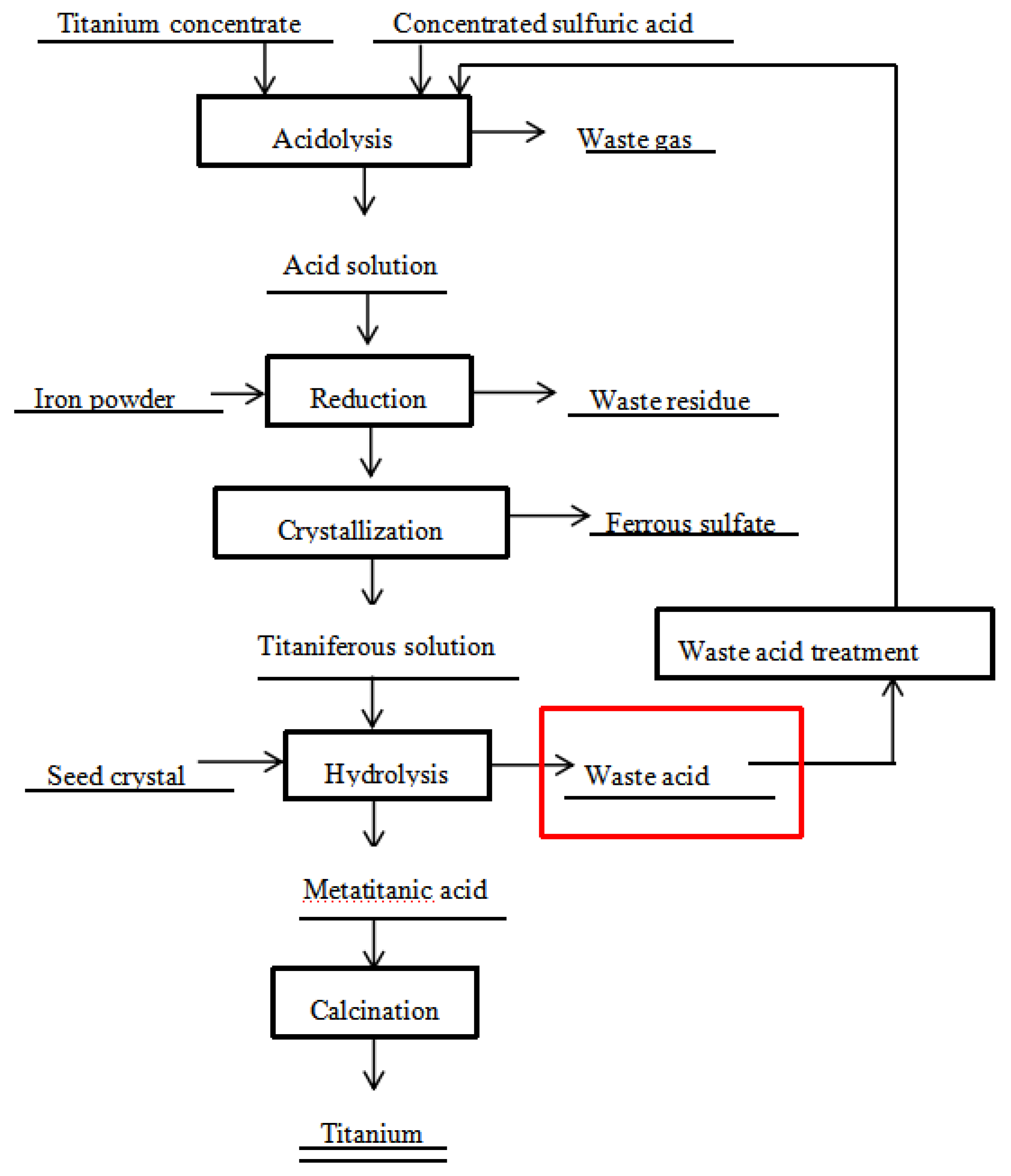
Metals | Free Full-Text | Preparation of Doped Iron Phosphate by Selective Precipitation of Iron from Titanium Dioxide Waste Acid