What is carbon disulfide?. Carbon disulfide is also known as… | by KAKALI GHOSH , Teacher,blogger. M.Sc chemistry. | Medium
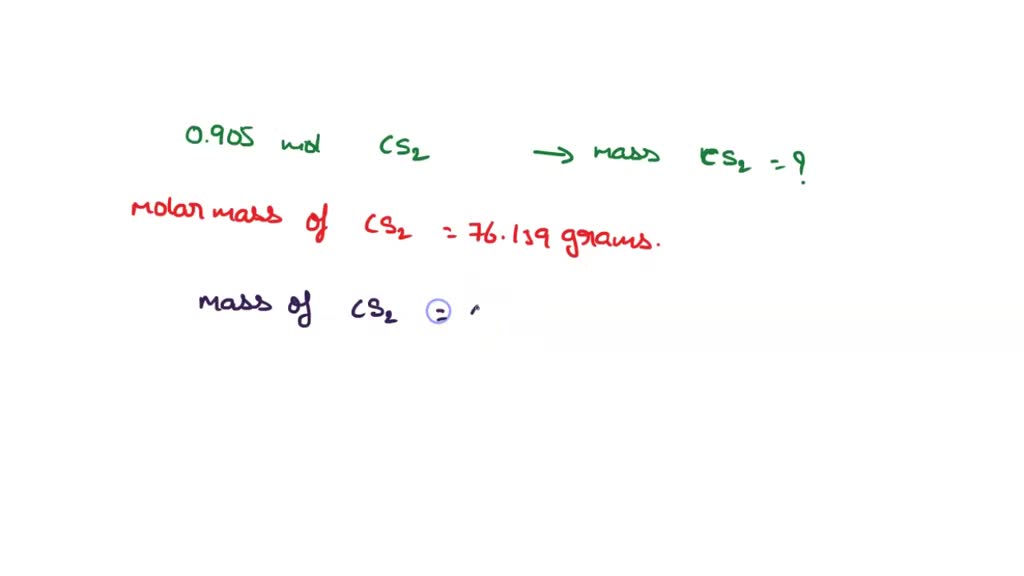
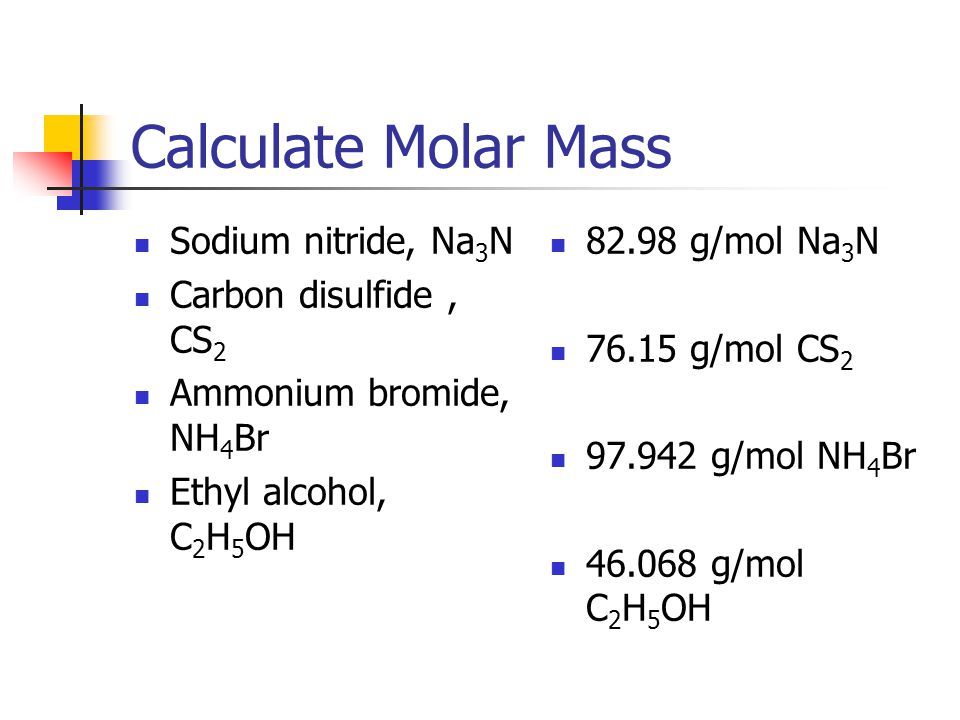
SOLVED: How many grams of carbon disulfide ( CS2 ) are there in 0.905 mol of the compound? 0.905 mol=

Carbon disulfide reacts with oxygen gas to produce carbon dioxide and sulfur dioxide, 1) Write the - brainly.com
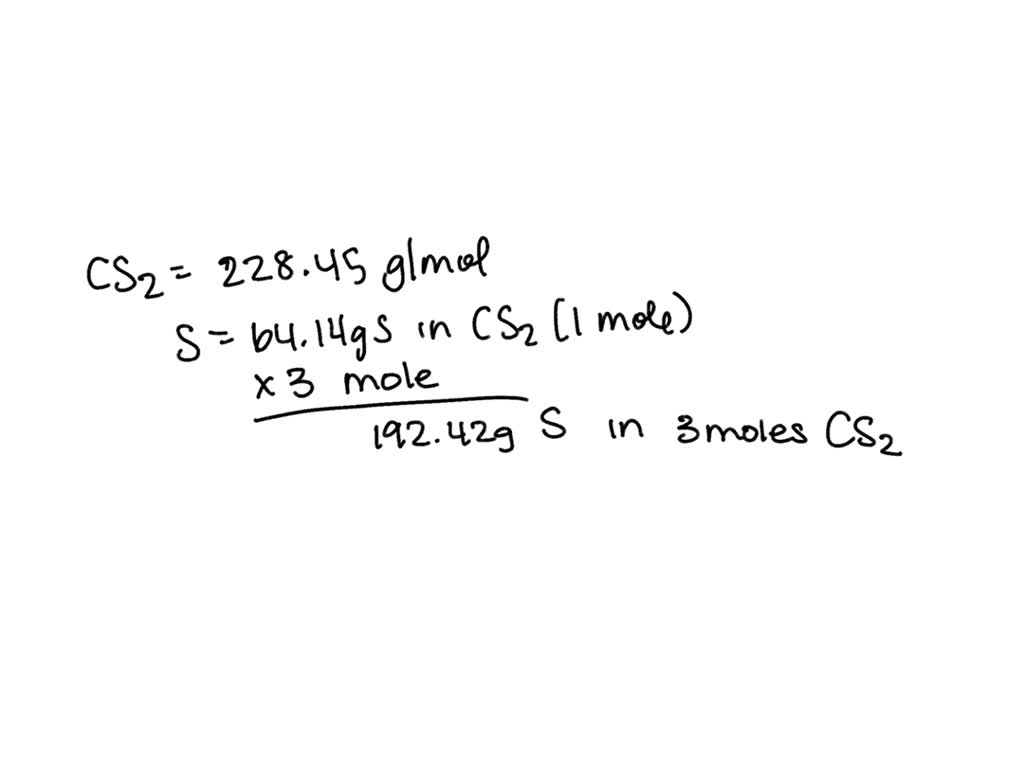
SOLVED: Question What is the mass of sulfur in 3.0 moles of carbon disulfide? 0 96.08 0 64.18 0 32.1 8 Question 8 What is the molar mass of barium hydroxide? 171.3 g/mole 154.3 g/mole 137.3 g/mole 308.6 g/mole 192 8

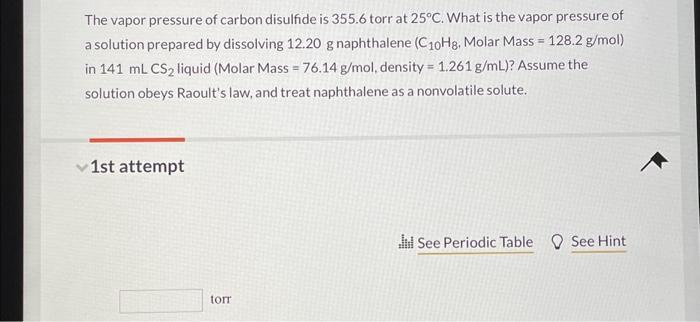
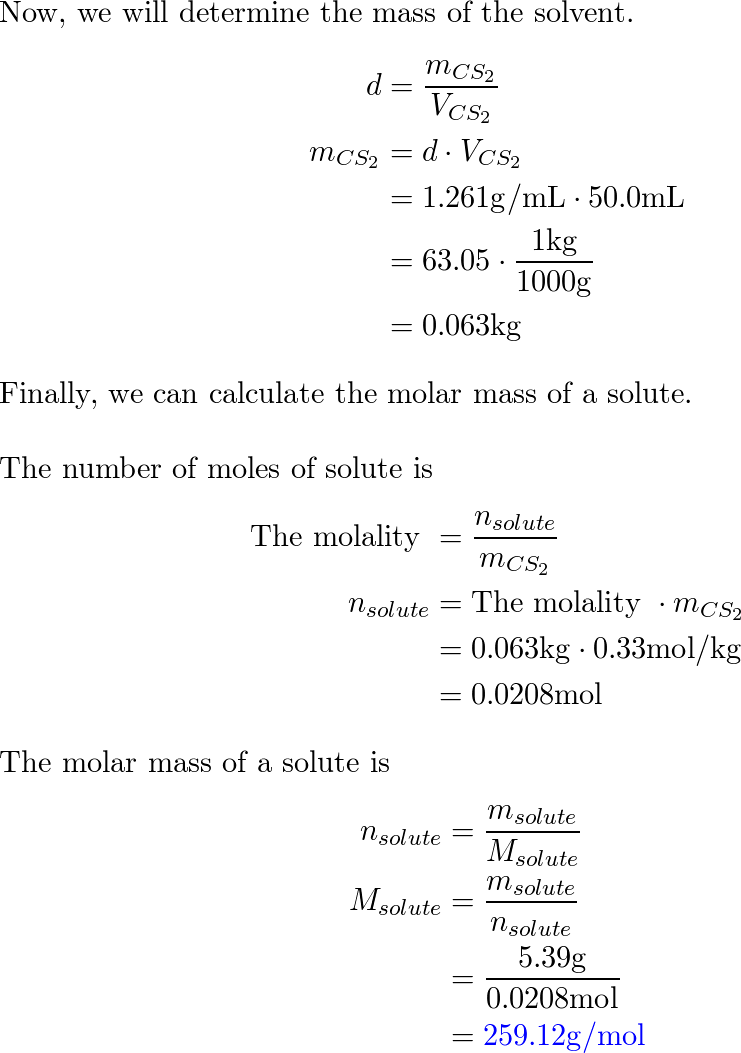
Free Online Help: for the above (carbon disulfide if 4.435 g of element are dissolve in 100.0 g of CS2 ) calcualte the atomic weight (or molar mass) of the unknown elements .

SOLVED: Given the balanced equation below. What mass of carbon disulfide ( CS2, molar mass = 76.1 g/mol) in grams must be oxidized to produce 32.0 g of SO2 (molar mass = 64.1

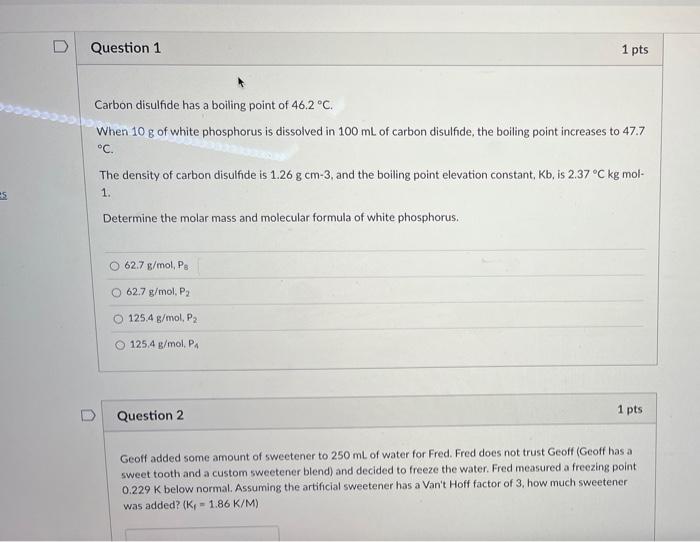
![ANSWERED] Determine the change in boiling point for 274 2 g of carbon - Kunduz ANSWERED] Determine the change in boiling point for 274 2 g of carbon - Kunduz](https://media.kunduz.com/media/sug-question-candidate/20220621004657969183-4426152.jpg)