science chemistry precipitation reaction carbon dioxide | Fundamental Photographs - The Art of Science

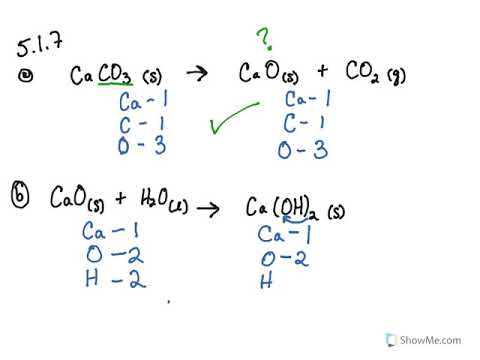
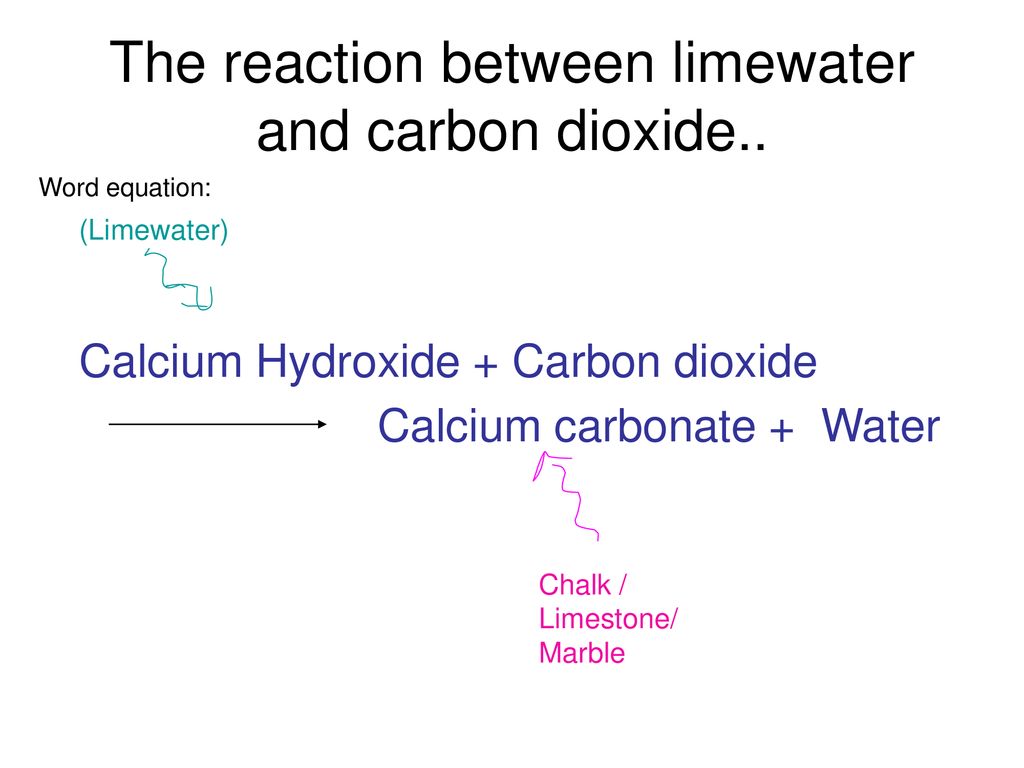
Write the balanced chemical equations for the following reaction:Calcium hydroxide + Carbon dioxide → Calcium Carbonate + Water.

Passing Carbon Dioxide Gas Through Calcium Hydroxide Solution Activity Diagram Drawing Easily - YouTube

Write the balanced chemical equations for the folowing reactions (a) Calcium hydroxide + Carbon - YouTube

Reactions of carbon dioxide - Gas chemistry - (CCEA) - GCSE Chemistry (Single Science) Revision - CCEA - BBC Bitesize

Carbon dioxide test. Test tube of limewater with carbon dioxide gas being bubbled through. The limewater, a solution of calcium hydroxide in water, is Stock Photo - Alamy

Look at Figure 4.1 and answer the following questions (a) What change would you observe in the calcium hydroxide solution taken - Question 53, 4. Carbon and its Compounds, Ncert Exemplar -

Ca(OH)2+CO2 =CaCO3 +H2O Balanced Equation|Calcium hydroxide+Carbon dioxide=Calcium carbonate + Water - YouTube
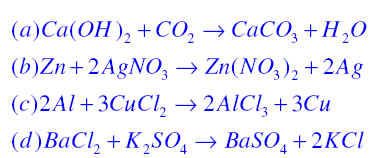
Write the balanced chemical equations for the following reactions. (a) Calcium hydroxide + Carbon dioxide → Calcium carbonate + Water | Pushpender86's Blog

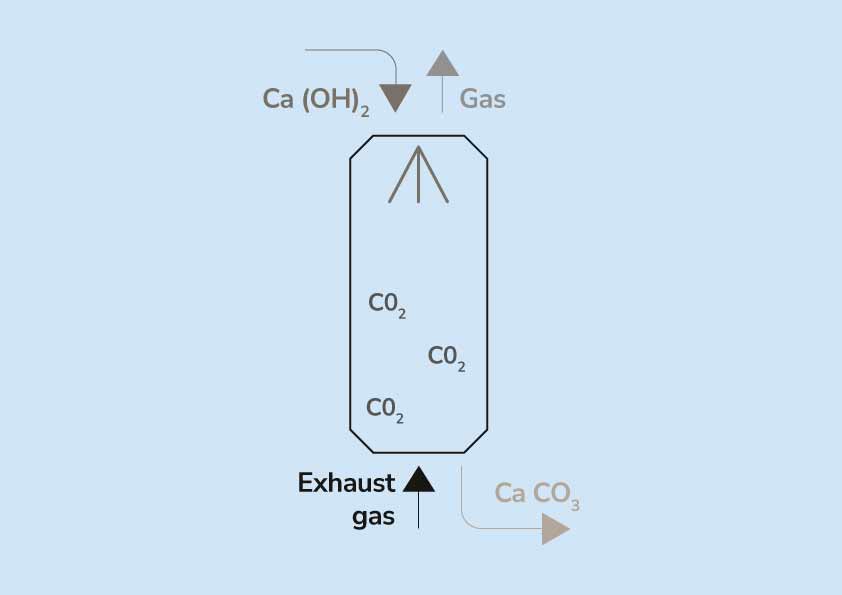
Energy-efficient chemical regeneration of AMP using calcium hydroxide for operating carbon dioxide capture process - ScienceDirect
Write the balanced chemical equations for the following reactions: i. Calcium hydroxide + Carbon dioxide → Calcium carbonate + Water - Sarthaks eConnect | Largest Online Education Community