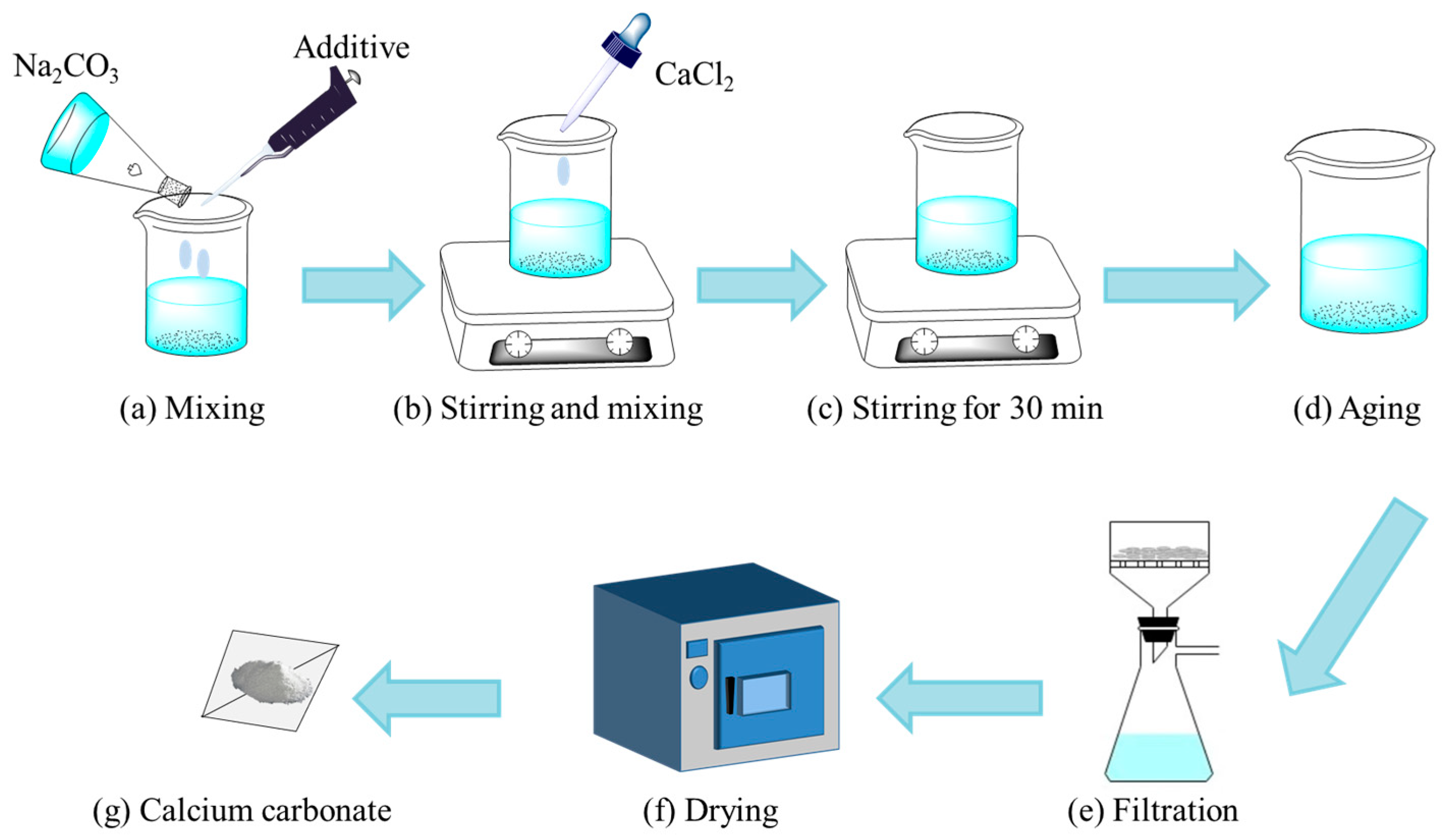
Crystals | Free Full-Text | Controllable Synthesis of Nano-Micro Calcium Carbonate Mediated by Additive Engineering
Decarbonisation of calcium carbonate at atmospheric temperatures and pressures, with simultaneous CO2 capture, through production of sodium carbonate - Energy & Environmental Science (RSC Publishing)
![PDF] Recovery of Sodium Hydroxide from Trona Ore and Calcium Carbonate as Raw Materials | Semantic Scholar PDF] Recovery of Sodium Hydroxide from Trona Ore and Calcium Carbonate as Raw Materials | Semantic Scholar](https://d3i71xaburhd42.cloudfront.net/c111a7b476ee6999fdd843657756ea6a4f670b83/4-Table2-1.png)
PDF] Recovery of Sodium Hydroxide from Trona Ore and Calcium Carbonate as Raw Materials | Semantic Scholar

The Effect of Variation Concentration Sodium Hydroxide (NaOH) on the Structure of Calcium Carbonate (CaCO3) Based on Natural Sand | Scientific.Net

Look figure 4.1 and answer the following question.(a) What change would you observe in the calcium hydroxide solution taken in tube B? (b) Write the reaction involved in test tubes A and

Bases. Jars containing calcium carbonate (Ca2CO3), copper oxide (CuO) and sodium hydroxide (NaOH). These compounds are classified as bases, because th Stock Photo - Alamy
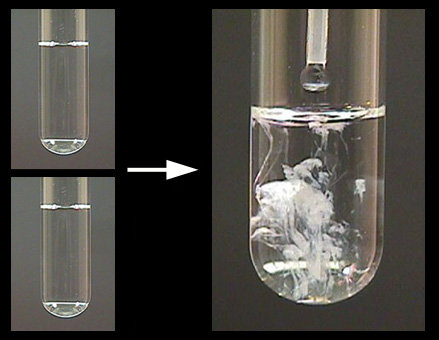
What happens when aqueous solutions of calcium chloride and of sodium carbonate are mixed? | Socratic
![PDF] Recovery of Sodium Hydroxide from Trona Ore and Calcium Carbonate as Raw Materials | Semantic Scholar PDF] Recovery of Sodium Hydroxide from Trona Ore and Calcium Carbonate as Raw Materials | Semantic Scholar](https://d3i71xaburhd42.cloudfront.net/c111a7b476ee6999fdd843657756ea6a4f670b83/4-Figure2-1.png)
PDF] Recovery of Sodium Hydroxide from Trona Ore and Calcium Carbonate as Raw Materials | Semantic Scholar
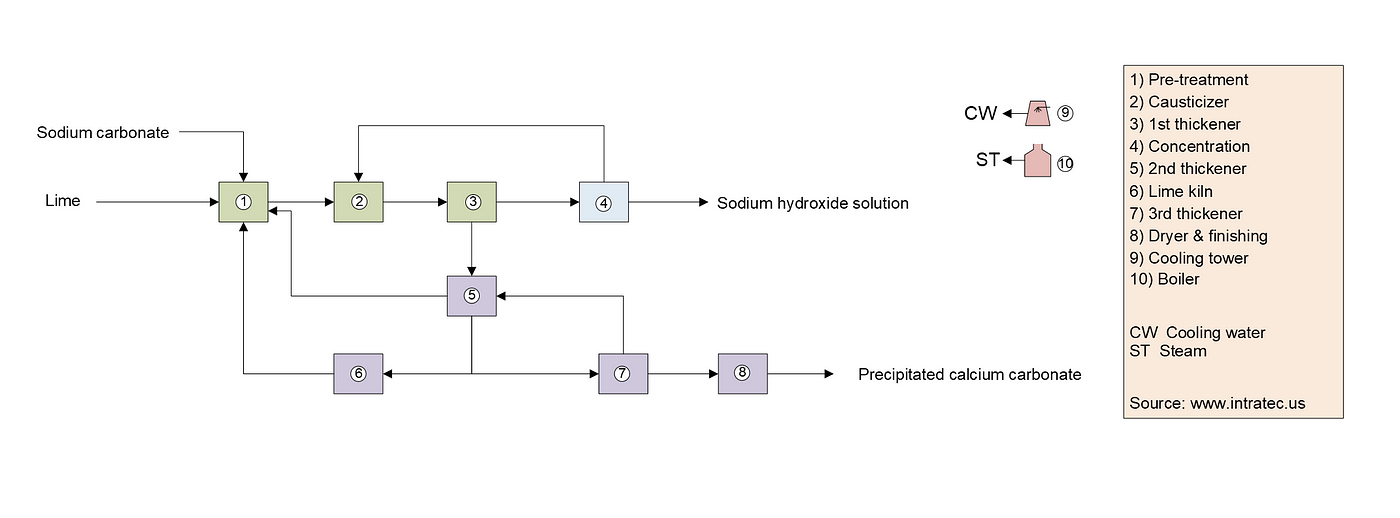
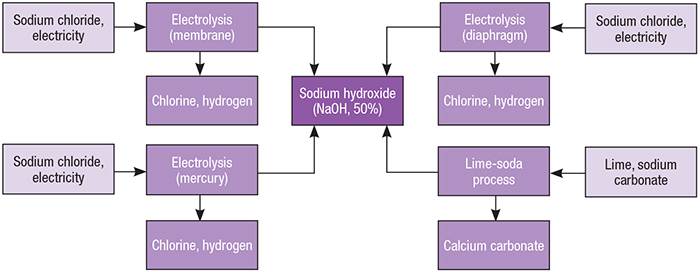
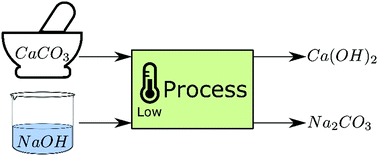
Sodium Hydroxide Production from Lime and Sodium Carbonate | Economic Analysis | by Intratec Solutions | Intratec Products Blog | Medium

The amount of sodium nitrate and sodium hydroxide in Kg with different... | Download Scientific Diagram
![PDF] Regeneration of Sodium Hydroxide from a Biogas Upgrading Unit through the Synthesis of Precipitated Calcium Carbonate: An Experimental Influence Study of Reaction Parameters | Semantic Scholar PDF] Regeneration of Sodium Hydroxide from a Biogas Upgrading Unit through the Synthesis of Precipitated Calcium Carbonate: An Experimental Influence Study of Reaction Parameters | Semantic Scholar](https://d3i71xaburhd42.cloudfront.net/00e03ec2cffecd61e840107d0d9fcb645c1e1c00/3-Figure1-1.png)
PDF] Regeneration of Sodium Hydroxide from a Biogas Upgrading Unit through the Synthesis of Precipitated Calcium Carbonate: An Experimental Influence Study of Reaction Parameters | Semantic Scholar

You are provided with: (i)an impure calcium carbonate labeled M (ii)Hydrochloric acid labeled solution N (iii)solution L containing 20g per litre sodium hydroxide

Figure 3 from Recovery of Sodium Hydroxide from Trona Ore and Calcium Carbonate as Raw Materials | Semantic Scholar
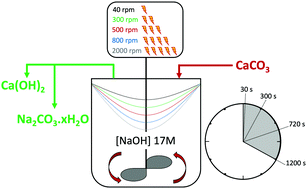
Decarbonisation of calcium carbonate in sodium hydroxide solutions under ambient conditions: effect of residence time and mixing rates - Physical Chemistry Chemical Physics (RSC Publishing)